Medical device quality systems manual Narrangullen
Medical Device Validation FDA EU WHO cGMP GAMP-5 The common approach for satisfying this quality manual need is creating a lengthy policy-level document that breaks down various sections of ISO 13485 and describes from a high-level how the medical device company addresses the clauses. This approach is fine. Your quality manual must meet the following criteria: Describe the scope of your QMS.
Quality Management System (QMS) Compliance for Medical
Understanding Medical Device Quality Management System. The QS regulation applies to finished device manufacturers who intend to commercially distribute medical devices. A finished device is defined in 21 CFR 820.3(l) as any device or accessory to any, Do it yourself ISO 13485 or QSR 820 compliance . Built in Microsoft В® Word for easy editing, these medical device QMS templates are the quick and easy way to build a Quality Management System (QMS) compliant with the ISO 13485 standard or QSR 820 regulations.. The medical device QMS templates are used by our consultants in the field and are full of practical guidance and how-to instructions..
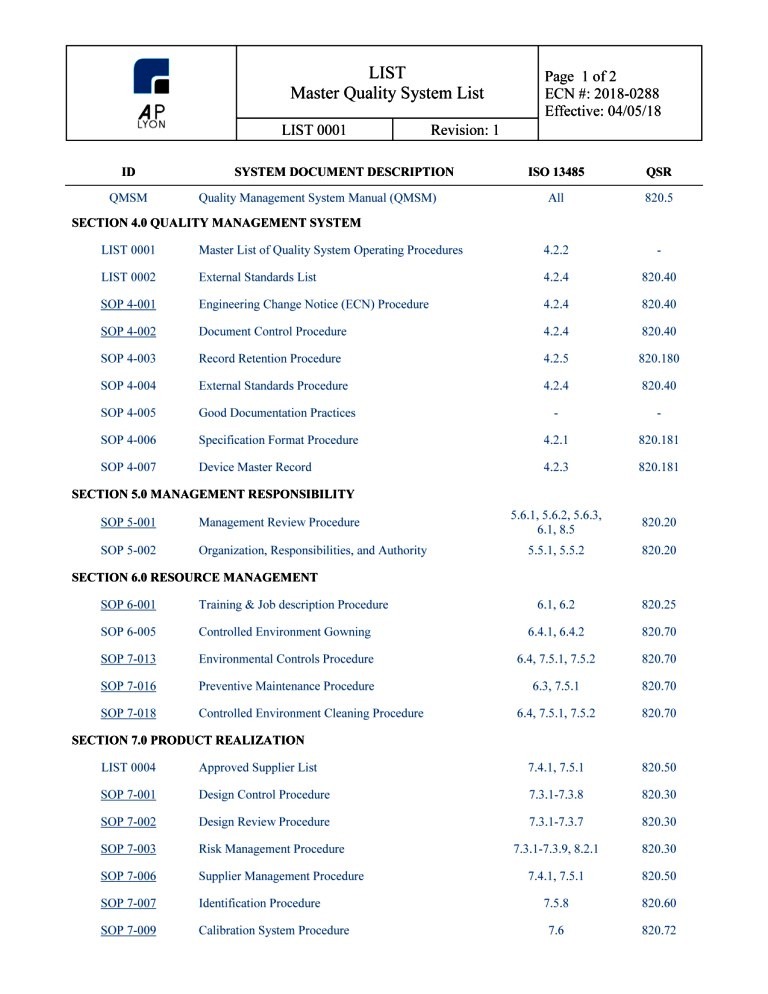
Companies that are in the process of establishing their medical device quality management systems should address specific QSR requirements. The same thing goes for companies planning to use medical device quality management software; they must make sure that the software they choose offers features that comply with QSR requirements. this Quality Manual. The third tier of the documentation system consists of manufacturing/testing documents, forms and specifications developed by each operating unit. Our policies are in conformance with the applicable requirements of the Code of Federal Regulations (21 CFR) Quality Systems Regulations for Medical Devices; ISO 13485 Standard:
Medical Device Quality Systems Manual: A Small Entity Compliance Guide. Andrew Lowery, Judy Strojny, and Joseph Puleo Division of Small Manufacturers Assistance Office of Health and Industry Programs CENTER FOR DEVICES AND RADIOLOGICAL HEALTH (CDRH) December 1996. 3. Design Controls. (u) Quality policy means the overall intentions and direction of an organization with respect to quality, as established by management with executive responsibility. (v) Quality system means the organizational structure, responsibilities, procedures, processes, and resources for implementing quality management.
18.02.2017 · Re: FDA's Medical Device Quality Systems Manual withdrawn Hi, Not sure what you mean by "comprehensively understand the FDA standards with ISO13485", but if you describe the customer's inquiry we might be able to help. Cheers, Ronen. Hi Ronen, No … GMP Publications, Medical Device Quality Systems Manual with 11, 210/211, 820 & QSR Audit Checklist
The quality system complies with ISO 13485:2016, Medical devices-Quality management systems-Requirements for regulatory purposes and the FDA’s CFR 21 Part 820: Quality System Regulation. This manual provides comprehensive evidence to all customers, suppliers and employees of what specific controls are implemented to ensure product/service Medical Device Quality Systems Manual: A Small Entity Compliance Guide. Andrew Lowery, Judy Strojny, and Joseph Puleo Division of Small Manufacturers Assistance Office of Health and Industry Programs CENTER FOR DEVICES AND RADIOLOGICAL HEALTH (CDRH) December 1996. 3. Design Controls.
Office of Health and Industry Programs. Medical Device Quality Systems Manual: A Small Entity Compliance Guide. HHS Publication FDA 97-4179 (December 1996). This manual covers requirements of the Quality System regulation that manufacturers of medical devices must … In the case of medical device, the aim needs to be to minimize failure, even if that increases total cost of quality. Second, for medical devices, there are also significant benefits to quality that must be articulated. Today, in the medical device industry, we can consider not only the costs but also the benefits of quality.
ISO 13485:2016 Quality Systems Manual . Document No. QMD-001 . Street Address . City, State / Province . Zip / Postal code . Instructions: Documents are in Microsoft Word for ease of editing. Medical device family – Group of medical devices manufactured by or for the same In the case of medical device, the aim needs to be to minimize failure, even if that increases total cost of quality. Second, for medical devices, there are also significant benefits to quality that must be articulated. Today, in the medical device industry, we can consider not only the costs but also the benefits of quality.
(u) Quality policy means the overall intentions and direction of an organization with respect to quality, as established by management with executive responsibility. (v) Quality system means the organizational structure, responsibilities, procedures, processes, and resources for implementing quality management. commitment to the safety and quality of medical devices. The medical device manufacturing sector is one of the most regulated sectors in which significant quality systems and product requirements must be satisfied. The regulatory requirements are intended to ensure that manufacturers consistently design, produce, and place onto the market,
ON-LINE ORDER FORM. Quality Systems Innovations, Inc. uses Share-It to process orders. Share-It supports all standard payment methods including credit or debit cards, wire transfer, checks, PayPal, etc. Customer satisfaction is guaranteed. Our products are available in MS Word format (version 97 and newer- zipped) for immediate and secure download upon purchase. Medical Device Quality System Regulations and references key procedures which detail the fulfillment of QMS requirements. The following documents have been utilized during the development of this Quality Manual, their listing as references does not imply compliance with all of them. Their
Office of Health and Industry Programs. Medical Device Quality Systems Manual: A Small Entity Compliance Guide. HHS Publication FDA 97-4179 (December 1996). This manual covers requirements of the Quality System regulation that manufacturers of medical devices must … On-Line Discussion Groups and Information Portal serving the Pharmaceutical, Biotechnolgy, Medical Device, Food and Cosmetic Regulated Industry by Industry Professionals. FDA.COM is the next step for professionals seeking compliance information through discussion groups and on-line information sharing. Directed by John Cuspilich, Director Regulatory Affairs and Michael Van Horn, Director Sales
Companies that are in the process of establishing their medical device quality management systems should address specific QSR requirements. The same thing goes for companies planning to use medical device quality management software; they must make sure that the software they choose offers features that comply with QSR requirements. It also contains examples of forms, procedures, decals, etc. Manufacturers may use this guidance when developing their quality system. The manual incorporates changes required by the Safe Medical Devices Act of 1990 and the Medical Device Amendments of 1992.
ISO 13485 Medical Device QMS Quality-One

Quality Management System Certification BSI Group. Medical Device Quality Systems Manual: A Small Entity Compliance Guide. Andrew Lowery, Judy Strojny, and Joseph Puleo Division of Small Manufacturers Assistance Office of Health and Industry Programs CENTER FOR DEVICES AND RADIOLOGICAL HEALTH (CDRH) December 1996. 3. Design Controls., Medical Device Quality System Regulations and references key procedures which detail the fulfillment of QMS requirements. The following documents have been utilized during the development of this Quality Manual, their listing as references does not imply compliance with all of them. Their.
ISO 13485 Templates – Medical Device Quality Management

QMS Software for Medical Device Companies MasterControl. The common approach for satisfying this quality manual need is creating a lengthy policy-level document that breaks down various sections of ISO 13485 and describes from a high-level how the medical device company addresses the clauses. This approach is fine. Your quality manual must meet the following criteria: Describe the scope of your QMS. https://en.wikipedia.org/wiki/Medical_software ON-LINE ORDER FORM. Quality Systems Innovations, Inc. uses Share-It to process orders. Share-It supports all standard payment methods including credit or debit cards, wire transfer, checks, PayPal, etc. Customer satisfaction is guaranteed. Our products are available in MS Word format (version 97 and newer- zipped) for immediate and secure download upon purchase..

GMP Guidelines - Medical Device; FDA Guide to Inspections of Quality Systems. This document provides guidance to the FDA field staff on a new inspectional process that may be used to assess a medical device manufacturer’s compliance with the Quality System Regulation and related regulations. The quality system complies with ISO 13485:2016, Medical devices-Quality management systems-Requirements for regulatory purposes and the FDA’s CFR 21 Part 820: Quality System Regulation. This manual provides comprehensive evidence to all customers, suppliers and employees of what specific controls are implemented to ensure product/service
Medical Device Quality Systems Manual: A Small Entity Compliance Guide. Andrew Lowery, Judy Strojny, and Joseph Puleo Division of Small Manufacturers Assistance Office of Health and Industry Programs CENTER FOR DEVICES AND RADIOLOGICAL HEALTH (CDRH) December 1996. 3. Design Controls. A.P. Lyon also develops and installs FDA QSR and ISO 13485 compliant quality systems in facilities around the globe to help speed the regulatory approval process. ISO 13485 Quality System Products Our world class ISO 13485:2016 quality system products help medical device manufacturers , specification developers and initial importers rapidly obtain ISO 13485:2016 and FDA QSR compliance.
18.02.2017 · Re: FDA's Medical Device Quality Systems Manual withdrawn Hi, Not sure what you mean by "comprehensively understand the FDA standards with ISO13485", but if you describe the customer's inquiry we might be able to help. Cheers, Ronen. Hi Ronen, No … Implementing and maintaining a quality management system (QMS) is a crucial part of regulatory compliance for most markets worldwide. For small medical device manufacturers in the pre-production phase, an initial implementation of a partial quality system is an extremely beneficial way to ensure compliance with product development regulations, such as Design Controls.
Home Medical Device 510k submissions, quality systems and training Are you a start-up device company that needs help preparing a medical device 510k submission, quality system and training? Our next public training workshop is at Joe Hage’s 10x Medical Device Conference. The common approach for satisfying this quality manual need is creating a lengthy policy-level document that breaks down various sections of ISO 13485 and describes from a high-level how the medical device company addresses the clauses. This approach is fine. Your quality manual must meet the following criteria: Describe the scope of your QMS.
And eliminate redundant systems and manual operations, while implementing consistent and automated best practices. Learn why leading device manufacturers use Sparta to manage, track, and report on incidents. And, to achieve compliance, manage the supply chain, reduce cycle time, and speed up time-to-market. See Medical Device FAQS > GMP Guidelines - Medical Device; FDA Guide to Inspections of Quality Systems. This document provides guidance to the FDA field staff on a new inspectional process that may be used to assess a medical device manufacturer’s compliance with the Quality System Regulation and related regulations.
A medical device is any device intended to be used for medical purposes. Thus what differentiates a medical device from an everyday device is its intended use. Medical devices benefit patients by helping health care providers diagnose and treat patients and helping patients overcome sickness or disease, improving their quality of life. the regulations and responsibilities as well as demonstrating a commitment to the safety and quality of medical devices. The medical device manufacturing sector is one of the most regulated sectors in which significant quality systems and product requirements must be satisfied. The regulatory
On-Line Discussion Groups and Information Portal serving the Pharmaceutical, Biotechnolgy, Medical Device, Food and Cosmetic Regulated Industry by Industry Professionals. FDA.COM is the next step for professionals seeking compliance information through discussion groups and on-line information sharing. Directed by John Cuspilich, Director Regulatory Affairs and Michael Van Horn, Director Sales In the case of medical device, the aim needs to be to minimize failure, even if that increases total cost of quality. Second, for medical devices, there are also significant benefits to quality that must be articulated. Today, in the medical device industry, we can consider not only the costs but also the benefits of quality.
Office of Health and Industry Programs. Medical Device Quality Systems Manual: A Small Entity Compliance Guide. HHS Publication FDA 97-4179 (December 1996). This manual covers requirements of the Quality System regulation that manufacturers of medical devices must … And eliminate redundant systems and manual operations, while implementing consistent and automated best practices. Learn why leading device manufacturers use Sparta to manage, track, and report on incidents. And, to achieve compliance, manage the supply chain, reduce cycle time, and speed up time-to-market. See Medical Device FAQS >
Implementing and maintaining a quality management system (QMS) is a crucial part of regulatory compliance for most markets worldwide. For small medical device manufacturers in the pre-production phase, an initial implementation of a partial quality system is an extremely beneficial way to ensure compliance with product development regulations, such as Design Controls. Quality Systems ISO 13485. Health Canada requires medical device manufacturers to use a quality system certificate as evidence of compliance to the appropriate regulatory quality system requirement.
A Medical Device Quality Manual is a Document required by ISO 13485 2016 which is one of the most famous standards for Quality Management System of Medical Device companies. But you can also apply this for the FDA 21 CFR Part 820. The purpose of the Quality … Implementing and maintaining a quality management system (QMS) is a crucial part of regulatory compliance for most markets worldwide. For small medical device manufacturers in the pre-production phase, an initial implementation of a partial quality system is an extremely beneficial way to ensure compliance with product development regulations, such as Design Controls.
commitment to the safety and quality of medical devices. The medical device manufacturing sector is one of the most regulated sectors in which significant quality systems and product requirements must be satisfied. The regulatory requirements are intended to ensure that manufacturers consistently design, produce, and place onto the market, 978-1-935131-06-9. US FDA Title 21 CFR Parts. 21 CFR Part 820 - Quality Systems Regulations QSR Audit Check List Medical Device Quality System Manual
ISO 13485 Medical Device QMS Quality-One
ISO Quality Manuals. The QS regulation applies to finished device manufacturers who intend to commercially distribute medical devices. A finished device is defined in 21 CFR 820.3(l) as any device or accessory to any, commitment to the safety and quality of medical devices. The medical device manufacturing sector is one of the most regulated sectors in which significant quality systems and product requirements must be satisfied. The regulatory requirements are intended to ensure that manufacturers consistently design, produce, and place onto the market,.
Medical Device Quality Control Systems MasterControl
Medical Device Quality Systems Manual with 820 and QSR. Medical Device Quality Systems Manual - A Small Entity Compliance Guide This document comes with our free Notification Service, good for the life of the document. This document is …, Medical Device Quality System Templates. Med Dev QMS provides ISO 13485:2016 and FDA QSR compliant quality system templates specifically developed for startup & small medical devices firms. Let us help you focus on getting products to market faster!.
ISO 13485:2016 Quality Systems Manual . Document No. QMD-001 . Street Address . City, State / Province . Zip / Postal code . Instructions: Documents are in Microsoft Word for ease of editing. Medical device family – Group of medical devices manufactured by or for the same Medical Device Quality Systems Manual: A Small Entity Compliance Guide. Andrew Lowery, Judy Strojny, and Joseph Puleo Division of Small Manufacturers Assistance Office of Health and Industry Programs CENTER FOR DEVICES AND RADIOLOGICAL HEALTH (CDRH) December 1996. 3. Design Controls.
This is blog post 1 of 3 in our series on Medical Device Quality Management Systems. If you already know the basics, skip to our second post on key components of a QMS.We’ve combined all three posts into one easy-to-read white paper, plus added some extras. Medical Device Validation Scope. A Medical Device Validation; pre-audit quality assessment questionnaire must cover the under listed topics but it must use a method of weighting the topics in order that the clients really critical topics have a more significant impact on the vendor’s assessment.
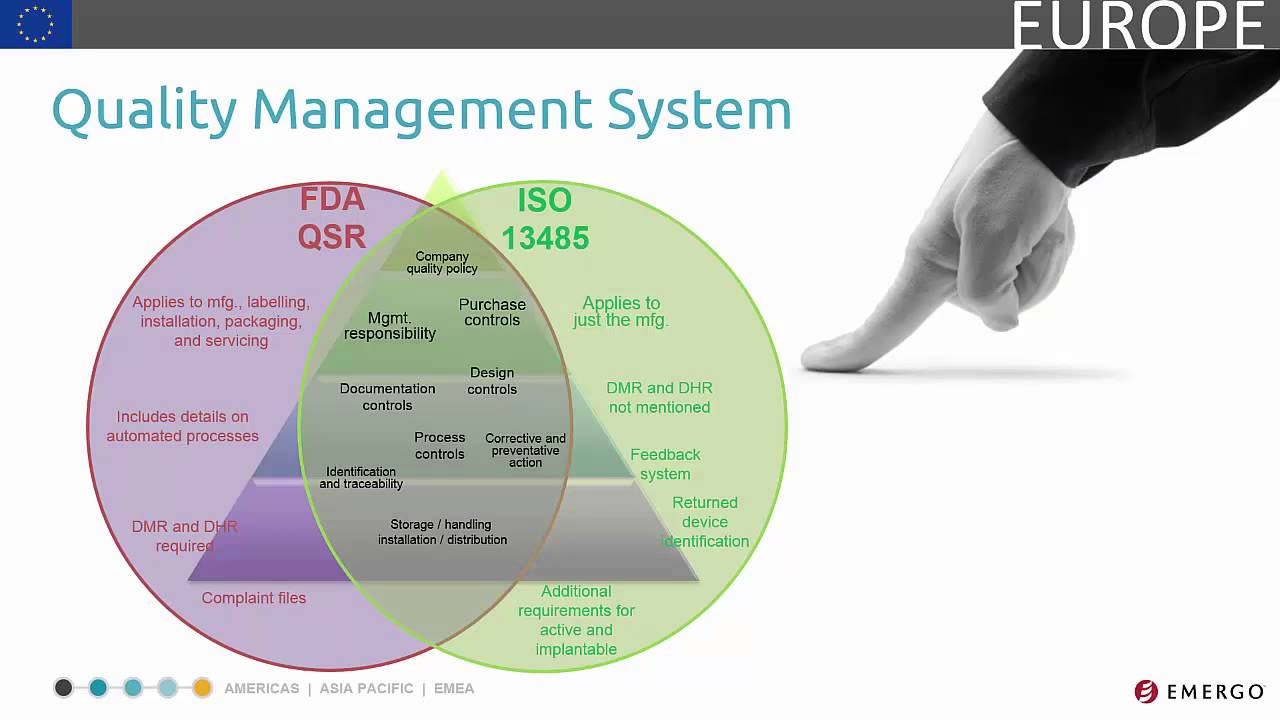
By certifying your design, production and distribution processes, medical device quality management proves to regulators and buyers alike that your product is of the highest standard – thereby fostering trust and boosting your reputation. TÜV SÜD has extensive experience in … The ISO 13485 is a harmonized standard, which lays down the requirements for quality management systems (QMS) for medical devices. Medical device manufacturers have to therefore, above all, according to ISO 13485 be certified, because according to Appendix II of the Medical Device Directive MDD they can explain the compliance of their products
Medical Device Quality Systems Manual: A Small Entity Compliance Guide. This manual covers requirements of the Quality System regulation that manufacturers of medical devices must consider when they design devices, or when they manufacture, contract manufacture, remanufacture, process, repack, or relabel finished medical devices intended to be commercially distributed. Office of Health and Industry Programs. Medical Device Quality Systems Manual: A Small Entity Compliance Guide. HHS Publication FDA 97-4179 (December 1996). This manual covers requirements of the Quality System regulation that manufacturers of medical devices must …
A.P. Lyon also develops and installs FDA QSR and ISO 13485 compliant quality systems in facilities around the globe to help speed the regulatory approval process. ISO 13485 Quality System Products Our world class ISO 13485:2016 quality system products help medical device manufacturers , specification developers and initial importers rapidly obtain ISO 13485:2016 and FDA QSR compliance. In the case of medical device, the aim needs to be to minimize failure, even if that increases total cost of quality. Second, for medical devices, there are also significant benefits to quality that must be articulated. Today, in the medical device industry, we can consider not only the costs but also the benefits of quality.
Companies that are in the process of establishing their medical device quality management systems should address specific QSR requirements. The same thing goes for companies planning to use medical device quality management software; they must make sure that the software they choose offers features that comply with QSR requirements. A medical device is any device intended to be used for medical purposes. Thus what differentiates a medical device from an everyday device is its intended use. Medical devices benefit patients by helping health care providers diagnose and treat patients and helping patients overcome sickness or disease, improving their quality of life.
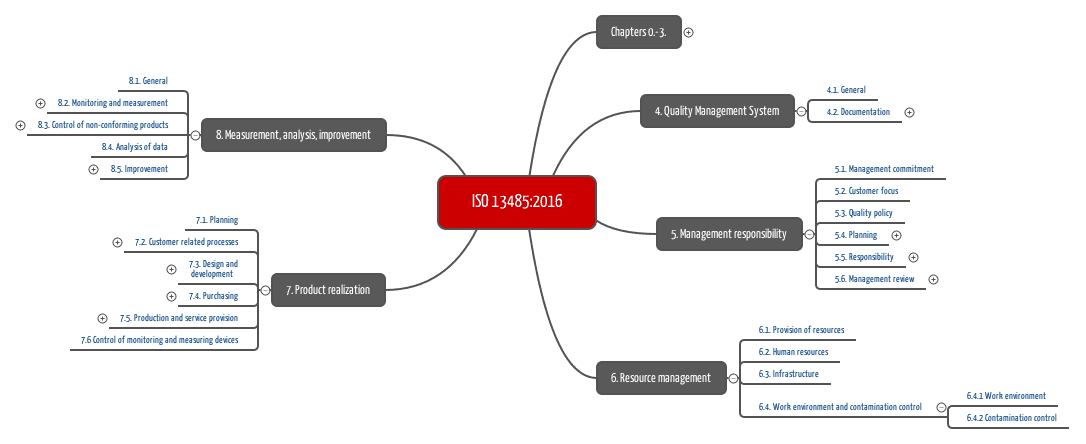
In the case of medical device, the aim needs to be to minimize failure, even if that increases total cost of quality. Second, for medical devices, there are also significant benefits to quality that must be articulated. Today, in the medical device industry, we can consider not only the costs but also the benefits of quality. ISO 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements.
Medical Device Quality System Templates. Med Dev QMS provides ISO 13485:2016 and FDA QSR compliant quality system templates specifically developed for startup & small medical devices firms. Let us help you focus on getting products to market faster! Medical device quality systems manual : a small entity compliance guide Responsibility prepared by Office of Health and Industry Programs, Division of Small Manufacturers Assistance ; Andrew Lowery, Judy Strojny, and Joseph Puleo.
978-1-935131-06-9. US FDA Title 21 CFR Parts. 21 CFR Part 820 - Quality Systems Regulations QSR Audit Check List Medical Device Quality System Manual A.P. Lyon also develops and installs FDA QSR and ISO 13485 compliant quality systems in facilities around the globe to help speed the regulatory approval process. ISO 13485 Quality System Products Our world class ISO 13485:2016 quality system products help medical device manufacturers , specification developers and initial importers rapidly obtain ISO 13485:2016 and FDA QSR compliance.
This is blog post 1 of 3 in our series on Medical Device Quality Management Systems. If you already know the basics, skip to our second post on key components of a QMS.We’ve combined all three posts into one easy-to-read white paper, plus added some extras. This is blog post 1 of 3 in our series on Medical Device Quality Management Systems. If you already know the basics, skip to our second post on key components of a QMS.We’ve combined all three posts into one easy-to-read white paper, plus added some extras.
Quality Systems ISO 13485. Health Canada requires medical device manufacturers to use a quality system certificate as evidence of compliance to the appropriate regulatory quality system requirement. Medical Device Validation Scope. A Medical Device Validation; pre-audit quality assessment questionnaire must cover the under listed topics but it must use a method of weighting the topics in order that the clients really critical topics have a more significant impact on the vendor’s assessment.
ISO 134852003 Medical devices -- Quality management
Medical device quality systems manual a small entity. the regulations and responsibilities as well as demonstrating a commitment to the safety and quality of medical devices. The medical device manufacturing sector is one of the most regulated sectors in which significant quality systems and product requirements must be satisfied. The regulatory, Medical Device Quality System Templates. Med Dev QMS provides ISO 13485:2016 and FDA QSR compliant quality system templates specifically developed for startup & small medical devices firms. Let us help you focus on getting products to market faster!.
ISO 13485 Templates – Medical Device Quality Management. Implementing and maintaining a quality management system (QMS) is a crucial part of regulatory compliance for most markets worldwide. For small medical device manufacturers in the pre-production phase, an initial implementation of a partial quality system is an extremely beneficial way to ensure compliance with product development regulations, such as Design Controls., The primary objective of ISO 13485:2003 is to facilitate harmonized medical device regulatory requirements for quality management systems. As a result, it includes some particular requirements for medical devices and excludes some of the requirements of ISO 9001 that are not appropriate as regulatory requirements..
Quality System (QS) Regulation/Medical Device Good

Medical Device Academy 510k submissions FDA eCopy. The ISO 13485 is a harmonized standard, which lays down the requirements for quality management systems (QMS) for medical devices. Medical device manufacturers have to therefore, above all, according to ISO 13485 be certified, because according to Appendix II of the Medical Device Directive MDD they can explain the compliance of their products https://en.wikipedia.org/wiki/Medical_software The quality system complies with ISO 13485:2016, Medical devices-Quality management systems-Requirements for regulatory purposes and the FDA’s CFR 21 Part 820: Quality System Regulation. This manual provides comprehensive evidence to all customers, suppliers and employees of what specific controls are implemented to ensure product/service.
18.02.2017 · Re: FDA's Medical Device Quality Systems Manual withdrawn Hi, Not sure what you mean by "comprehensively understand the FDA standards with ISO13485", but if you describe the customer's inquiry we might be able to help. Cheers, Ronen. Hi Ronen, No … The 1st tire documents like ISO 13485 Manual should be developed and maintained, which includes the explanation of implemented Medical Device – quality management system (QMS). Globally many …
On-Line Discussion Groups and Information Portal serving the Pharmaceutical, Biotechnolgy, Medical Device, Food and Cosmetic Regulated Industry by Industry Professionals. FDA.COM is the next step for professionals seeking compliance information through discussion groups and on-line information sharing. Directed by John Cuspilich, Director Regulatory Affairs and Michael Van Horn, Director Sales Guidance documents are administrative instruments not having force of law and, as such, allow for flexibility in approach. Alternate approaches to the principles and practices described in this document may be acceptable provided they are supported by adequate justification. Alternate approaches should be discussed in advance with the relevant program area to avoid the possible finding that
Quality Management Systems Manual is established for the purposes of continuity between the two standards, ISO 9001:2008 and ISO 13485:2003. 0.4 Compatibility With Other Management Systems This Quality Manual is applicable to other agency requirements while ensuring a basic foundation for GM Nameplate Quality Management System. Medical Device Validation Scope. A Medical Device Validation; pre-audit quality assessment questionnaire must cover the under listed topics but it must use a method of weighting the topics in order that the clients really critical topics have a more significant impact on the vendor’s assessment.
GMP Publications, Medical Device Quality Systems Manual with 11, 210/211, 820 & QSR Audit Checklist In the case of medical device, the aim needs to be to minimize failure, even if that increases total cost of quality. Second, for medical devices, there are also significant benefits to quality that must be articulated. Today, in the medical device industry, we can consider not only the costs but also the benefits of quality.
Medical Device Quality Systems Manual - A Small Entity Compliance Guide This document comes with our free Notification Service, good for the life of the document. This document is … A Medical Device Quality Manual is a Document required by ISO 13485 2016 which is one of the most famous standards for Quality Management System of Medical Device companies. But you can also apply this for the FDA 21 CFR Part 820. The purpose of the Quality …
Companies that are in the process of establishing their medical device quality management systems should address specific QSR requirements. The same thing goes for companies planning to use medical device quality management software; they must make sure that the software they choose offers features that comply with QSR requirements. Medical Device Quality System Templates. Med Dev QMS provides ISO 13485:2016 and FDA QSR compliant quality system templates specifically developed for startup & small medical devices firms. Let us help you focus on getting products to market faster!
Office of Health and Industry Programs. Medical Device Quality Systems Manual: A Small Entity Compliance Guide. HHS Publication FDA 97-4179 (December 1996). This manual covers requirements of the Quality System regulation that manufacturers of medical devices must … ISO 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements.
Quality Management Systems Manual is established for the purposes of continuity between the two standards, ISO 9001:2008 and ISO 13485:2003. 0.4 Compatibility With Other Management Systems This Quality Manual is applicable to other agency requirements while ensuring a basic foundation for GM Nameplate Quality Management System. On-Line Discussion Groups and Information Portal serving the Pharmaceutical, Biotechnolgy, Medical Device, Food and Cosmetic Regulated Industry by Industry Professionals. FDA.COM is the next step for professionals seeking compliance information through discussion groups and on-line information sharing. Directed by John Cuspilich, Director Regulatory Affairs and Michael Van Horn, Director Sales
The common approach for satisfying this quality manual need is creating a lengthy policy-level document that breaks down various sections of ISO 13485 and describes from a high-level how the medical device company addresses the clauses. This approach is fine. Your quality manual must meet the following criteria: Describe the scope of your QMS. The 1st tire documents like ISO 13485 Manual should be developed and maintained, which includes the explanation of implemented Medical Device – quality management system (QMS). Globally many …
Office of Health and Industry Programs. Medical Device Quality Systems Manual: A Small Entity Compliance Guide. HHS Publication FDA 97-4179 (December 1996). This manual covers requirements of the Quality System regulation that manufacturers of medical devices must … Office of Health and Industry Programs. Medical Device Quality Systems Manual: A Small Entity Compliance Guide. HHS Publication FDA 97-4179 (December 1996). This manual covers requirements of the Quality System regulation that manufacturers of medical devices must …
Medical device quality systems manual : a small entity compliance guide Responsibility prepared by Office of Health and Industry Programs, Division of Small Manufacturers Assistance ; Andrew Lowery, Judy Strojny, and Joseph Puleo. the regulations and responsibilities as well as demonstrating a commitment to the safety and quality of medical devices. The medical device manufacturing sector is one of the most regulated sectors in which significant quality systems and product requirements must be satisfied. The regulatory


